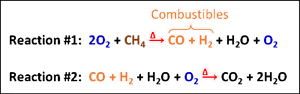The four critical measurements of combustion analyzers
Combustion measurements are critical to ensure the safe and efficient operation of fired equipment. These measurements provide the information needed to make key decisions, such as setting air/fuel ratios at the burner, lowering excess air setpoints, detecting the presence of carbon monoxide breakthrough and alerting accumulation of a fuel-rich mixture. Each of the following critical measurements equip operators with the critical information needed to fully monitor and safely operate fired equipment.
Combustion Fundamentals
Before we dive into measurements, we must first consider some of the fundamentals of combustion. To start, the Fire Triangle summarizes the three elements needed to ignite and sustain a fire: oxygen, fuel, and heat (or energy in the form of a spark). If any of the elements are removed, the flame goes out. Likewise, oxygen and fuel can mix at a burner tip, but without an initial spark, they do not react to form a flame.
The Fire Triangle
In addition, the reactions of combustion provide additional context for monitoring combustion. Under perfect conditions, hydrocarbon fuels, such as methane, react to form carbon dioxide (CO2) and water (H2O). However, in practice, combustion is never perfect and there are always small amounts of combustibles present in the flue gas, often taking the form of ppm-levels of carbon monoxide (CO) and hydrogen (H2). Only in cases when burner the burner is starved of oxygen or operating air-lean (fuel-rich) could the combustibles level rise high enough to signal a potentially unsafe condition.
Simplified sub-reactions of the combustion of oxygen (O2) and methane (CH4)
Both the Fire Triangle and the sub-reactions of combustion provide valuable insight and context for each of the following four critical combustion measurements.
- Oxygen Measurement (O2)
The first, most critical, measurement in combustion is oxygen. Oxygen is one of the three key elements of the Fire Triangle. It provides a critical operating setpoint for combustion air blowers.
There are two types of oxygen measurement in practice: gross oxygen and net oxygen. Gross oxygen represents the exact amount of oxygen in the flue gas, regardless of any other constituents. Net oxygen measurement represents the residual or excess oxygen in the flue gas, after all combustible compounds are consumed.
During normal operation, the difference between gross and net oxygen measurements is negligible. However, during an upset case, they respond differently as a net oxygen reading would decrease as it consumes the incoming combustible material while the gross oxygen measurement would remain rather flat and unchanged. This distinction has made net oxygen measurements more readily implementable for setting burner air/fuel ratios as its readings account for any unburnt content within the flue gas and directly correlate with excess air levels at the burner.
Historically, heater and boiler manufacturers have used net oxygen measurements to set their air/fuel ratios and ensure safe excess air levels within the combustion chamber. Certain technologies, such as zirconium oxide, provide this net oxygen measurement very reliably.
- Combustibles Measurement (CO+H2)
The second most critical measurement is combustibles. These measurements play two very important roles in industry: safety monitoring and process efficiency.
For safety monitoring, combustibles measurements detect and signal the onset of incomplete combustion. Catalytic detectors can provide a ppm-level indication of the combined carbon monoxide (CO) and hydrogen (H2) in the process stream – in a single umbrella reading – again, known as the “combustibles measurement.” Other approaches monitor for CO alone. In either case, the combustibles measurement provides a method to monitor for both CO breakthrough and insufficient levels of air at the burner.
From an efficiency standpoint, the combustibles measurement provides a secondary reference point to allow operators to lower their combustion air levels at the burner. Oxygen measurement alone provides an operating setpoint, but the combustibles measurement gives operators additional information to lower excess air levels safely – BEFORE reaching CO breakthrough. Lower excess air levels mean less extra air to heat and thus, less fuel consumed at the burner.
- Methane & Hydrocarbon Measurement (CH4+)
While you would expect methane (CH4) and hydrocarbon fuels to fully combust in a hot firebox, it is possible for them to gradually accumulate from a flame-out during normal operation or fuel leak during start-up. A methane and hydrocarbon measurement provides an additional layer of visibility to safely detect the accumulation of a fuel-rich mixture.
Similar to the combustibles detectors, methane/hydrocarbon detectors are also catalytic in nature and provide an umbrella, all-in-one measurement. However, unlike the combustibles detector, methane detectors operate much hotter. Methane is the hardest hydrocarbon to crack, and the methane detector operates at a high enough temperature to crack the methane molecule and provide precise percent-level measurements. Note that methane detectors are also capable of detecting other hydrocarbons (such as ethane, propane, butane), according to their specific reactivities.
Above all, the methane/hydrocarbon measurement provides added monitoring to detect unburnt fuels, process tube leaks and loss of flame during start-up and normal operation.
- Indication of Process Representation
Beyond the measurement of oxygen, combustibles and methane/hydrocarbon, it is important to ensure that these measurements are representative of the process itself. The fourth critical measurement is exactly that: indication of a process representation. This fourth measurement can vary widely depending on the combustion analyzer type, measurement technologies and sample system used, but its importance is emphasized because this indication justifies the relevance of the other three measurements.
In an extractive analyzer, it is important to make sure that the sampling system is not plugged or fouled by particulate. Plugging would reduce the volume of sample gas sent to the detectors and be less representative of the process itself. In this case, a flow sensor would provide that fourth measurement to ensure a large enough sample is taken to be representative of the process. A flow sensor would also provide the ability to alarm for any potential concerns of plugging.
Other technologies and arrangements may depend on a temperature or pressure measurement to ensure the measurement is representative of the process. In these cases, it would be critical to have this information available at all times to ensure the validity of the oxygen, combustibles and methane/hydrocarbons readings.
Summary
As a brief recap, there are four critical measurements for any combustion analyzer. First, an oxygen measurement provides a setpoint to operate the fired equipment and set the air/fuel ratio at the burner. Second, a combustibles measurement provides a safety mechanism for monitoring for CO breakthrough and also an optimization point to reduce excess air levels and thereby, reduce fuel consumption. Third, the methane/hydrocarbons measurement provides an additional safeguard to monitor and detect flame out & gas leaks during start-up and normal operation. Fourth, an indication of process representation is important to ensure the relevance of the other three measurements, and this can take the form of a sample flow measurement or even a temperature or pressure measurement, depending on the technologies and arrangement of the combustion analyzer used. All four of these measurements provide operators with a broader picture to fully monitor and safely operate their fired heater equipment.
Learn about AMETEK Thermox combustion analyzers and the solutions we offer.